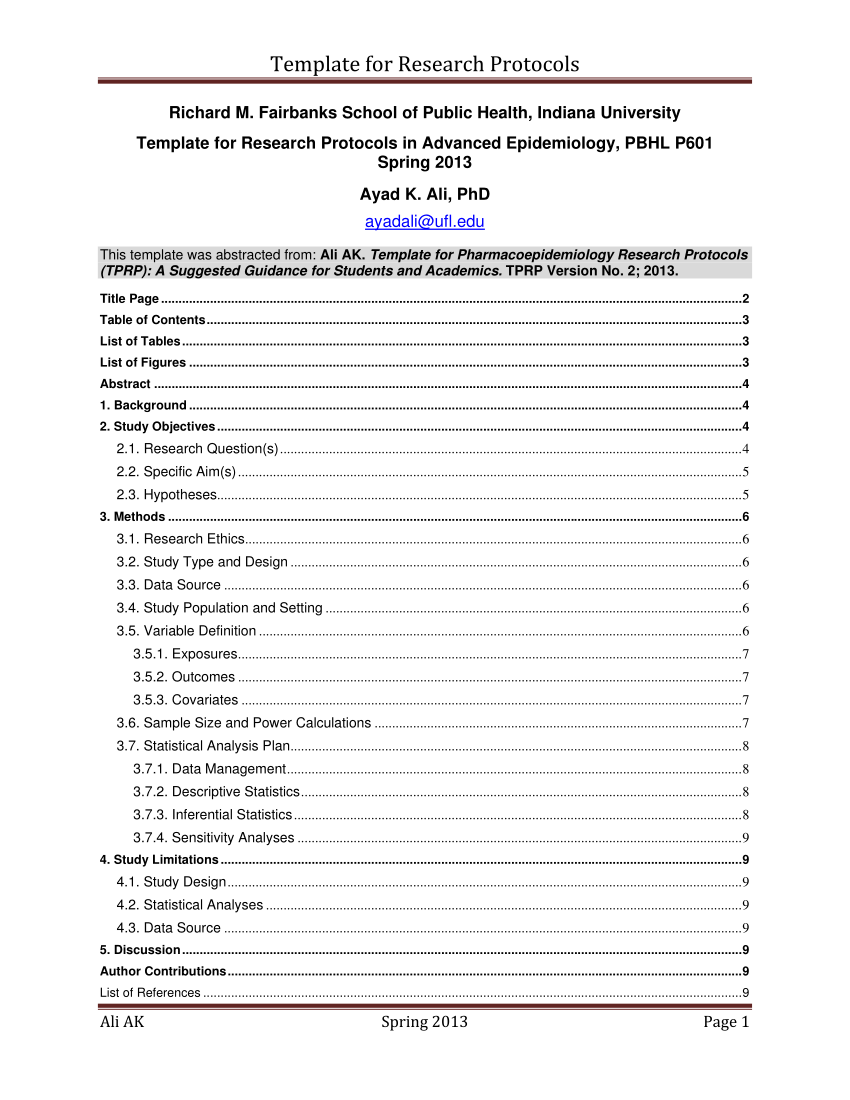
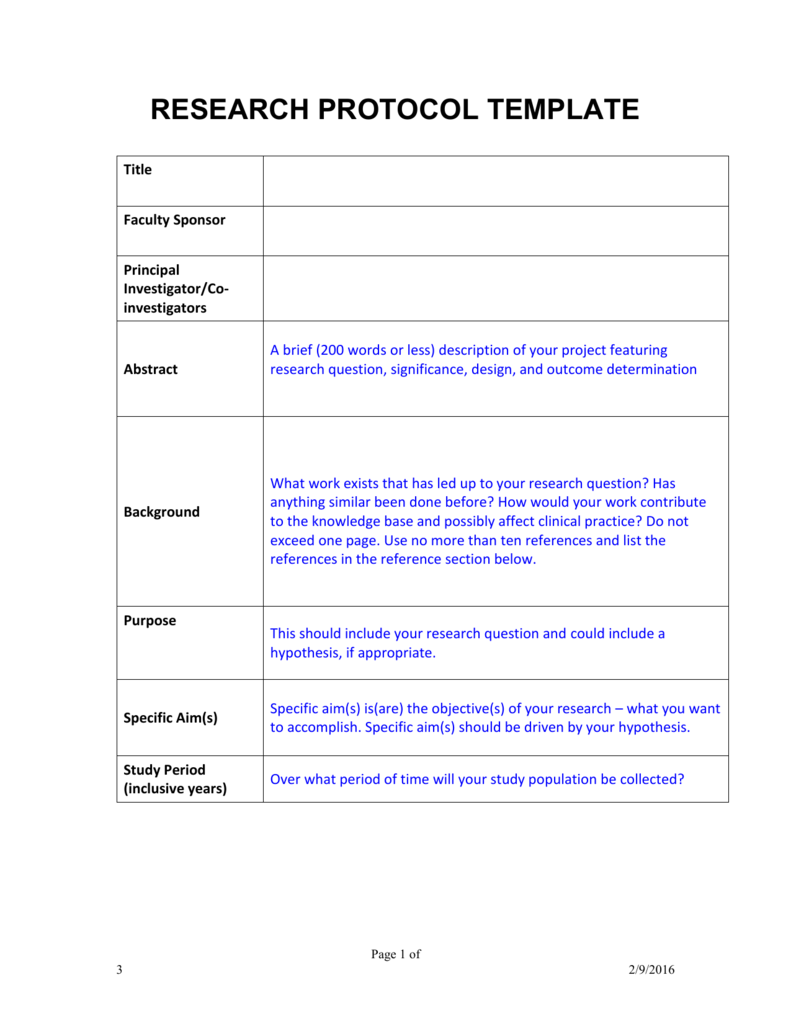
Research Protocol Template
Research Protocol Template - Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web the irb office has developed protocol templates for use by the northwestern university research community to. Web the irb provides several protocol templates on this page. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. They follow the format of typical nih and industry. Web this page includes seven different protocol templates for developing a variety of different new research.
Web this page includes seven different protocol templates for developing a variety of different new research. Web the irb provides several protocol templates on this page. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. They follow the format of typical nih and industry. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web the irb office has developed protocol templates for use by the northwestern university research community to.
Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. They follow the format of typical nih and industry. Web the irb office has developed protocol templates for use by the northwestern university research community to. Web the irb provides several protocol templates on this page. Web this page includes seven different protocol templates for developing a variety of different new research.
(PDF) Template for Research Protocols in Advanced Epidemiology
Web this page includes seven different protocol templates for developing a variety of different new research. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web the irb provides several protocol templates on this page. Web the irb office has developed protocol templates for use by the northwestern university research.
research protocol template
Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web this page includes seven different protocol templates for developing a variety of different new research. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web the irb provides several.
Nih Protocol Template Master of Documents
Web the irb provides several protocol templates on this page. They follow the format of typical nih and industry. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web this page includes seven different protocol templates for developing a variety of different new research. Web the irb office has.
Minimal risk research protocol template in Word and Pdf formats page
Web the irb provides several protocol templates on this page. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web this page includes seven different protocol templates for developing a.
Research Protocol Template PDF Risk Survey Methodology
Web the irb provides several protocol templates on this page. Web the irb office has developed protocol templates for use by the northwestern university research community to. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web nih applicants can use a template with instructional and sample text to help.
Template Device protocol
Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web this page includes seven different protocol templates for developing a variety of different new research. They follow the format of typical nih and industry. Web cite permissions share abstract a study protocol is an important document that specifies the.
Research protocol template
They follow the format of typical nih and industry. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web the irb office has developed protocol templates for use by the northwestern university research community to. Web nih applicants can use a template with instructional and sample text to help write.
Template for a Case Study Protocol
Web the irb office has developed protocol templates for use by the northwestern university research community to. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. They follow the format of typical nih and industry. Web cite permissions share abstract a study protocol is an important document that specifies.
(PDF) HUMAN RESEARCH PROTOCOL TEMPLATE (Only for Non Exempt minimal
Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web the irb office has developed protocol templates for use by the northwestern university research community to. Web the irb provides several protocol templates on this page. Web cite permissions share abstract a study protocol is an important document that.
Minimal risk research protocol template in Word and Pdf formats page
They follow the format of typical nih and industry. Web the irb provides several protocol templates on this page. Web the irb office has developed protocol templates for use by the northwestern university research community to. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web this page includes seven.
Web The Irb Office Has Developed Protocol Templates For Use By The Northwestern University Research Community To.
They follow the format of typical nih and industry. Web cite permissions share abstract a study protocol is an important document that specifies the research plan for a. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2. Web this page includes seven different protocol templates for developing a variety of different new research.